Special metal selection for plate heat exchangers.(HY-industry technical centre)
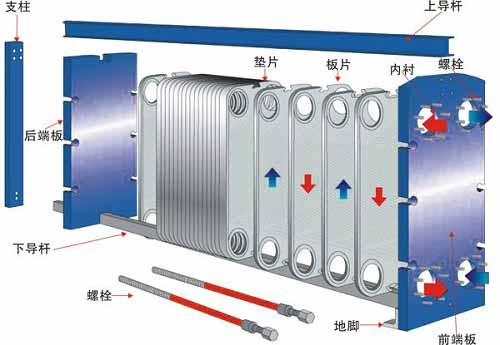
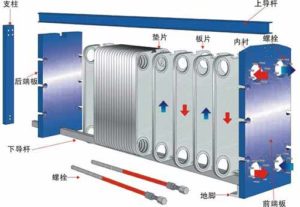 As is well known, plate heat exchangers have high heat transfer efficiency, large logarithmic temperature difference, light weight, small footprint, convenient detachable cleaning, and easy to change the heat exchange area or process combination, and are suitable for multiple medium heat exchange.
As is well known, plate heat exchangers have high heat transfer efficiency, large logarithmic temperature difference, light weight, small footprint, convenient detachable cleaning, and easy to change the heat exchange area or process combination, and are suitable for multiple medium heat exchange.
For the life of the equipment, HY-industry believes that an important factor is to test the corrosion conditions, to ensure the stable operation of the equipment, it is more important to choose the appropriate anti-corrosion equipment materials.
For plate heat exchangers, the side pressure plates and inlet and outlet ports at both ends are usually made of carbon steel, while the inner plates are usually pressed with stainless steel or corrosion-resistant alloy sheets of 0.5 to 0.8 mm thick (in special cases, 1 mm).
The cooling water is generally a local water source. Because the chloride ion in the water will cause different degrees of corrosion to stainless steel and alloy, the medium to be cooled is highly corrosive with respect to chemical equipment. Therefore, the plate material of the plate heat exchanger is The choice depends not only on the amount of chloride ions in the circulating cooling water, but also on the corrosion parameters of the medium. Of course, the temperature and the pH of the water are also the main factors that determine the appropriate material for the corrosion resistance of the inner sheet.
1. Three major corrosion analysis common to plate heat exchangers.
-
 There are many kinds of corrosion, and the form of metal corrosion can be generally divided into uniform corrosion and local corrosion. The former occurs more uniformly on the entire surface of the metal, and the latter only occurs locally. Typical local corrosion is: intergranular corrosion, pitting corrosion, crevice corrosion, stress corrosion, galvanic corrosion, erosion corrosion, corrosion fatigue, delamination corrosion. The circulating cooling water with the presence of chloride ions mainly causes corrosion, stress corrosion and crevice corrosion to the plate heat exchanger.
There are many kinds of corrosion, and the form of metal corrosion can be generally divided into uniform corrosion and local corrosion. The former occurs more uniformly on the entire surface of the metal, and the latter only occurs locally. Typical local corrosion is: intergranular corrosion, pitting corrosion, crevice corrosion, stress corrosion, galvanic corrosion, erosion corrosion, corrosion fatigue, delamination corrosion. The circulating cooling water with the presence of chloride ions mainly causes corrosion, stress corrosion and crevice corrosion to the plate heat exchanger. -
In the plate heat exchanger, the inner surface of the inner plate is generally covered with a protective passivation film, which is slightly corroded, but the metal exposed by the micro-shock is caused by defects on the surface (such as scratches, non-metallic inclusions, etc.). As a battery anode, the membrane around the enlarged area becomes the cathode, which causes local severe corrosion points.
-
For plate heat exchangers, stress is generated when the inner plate is pressed. Therefore, if the inner plate is made of austenitic stainless steel, the environment in which chloride ions are present will cause typical stress corrosion.
-
Crevice corrosion is a special form of pitting corrosion that occurs in the gap and is in the form of a groove. For the plate heat exchanger, the inner plate is sealed by the plates pressed together by a gasket. The two plates have a sealing groove on one side and the other surface is generally flat. The gasket and the plate after pressing There will be gaps between them. In the gap is an anoxic zone, the pH value in the slit decreases, and the chloride ion concentration increases, thereby accelerating corrosion. Therefore, crevice corrosion is also a typical corrosion of Special metal plate heat exchangers.
-
HY-industry believes that pitting corrosion, stress corrosion and crevice corrosion are present in the failure of the plate heat exchanger to varying degrees due to the surface of the plate, the pressing process and the presence of contact points.Corrosion is a chemical reaction, and the rate of corrosion increases by about 1 to 3 times for every 10 temperatures. Generally, the corrosion rate always increases with increasing temperature, the temperature rises, the diffusion speed increases, and the electrolyte resistance decreases, which accelerates the reaction of the corroded battery. Although there are occasional exceptions, HY-industry thinks it is very rare and does not do too much analysis here.
2. Scientific selection of various Special metal materials suitable for chemical environment.
-
 As a material choice for the plate heat exchanger plate, if the cooling medium is ignored, only the chloride ion and temperature combination of the cooling water need to be considered. It is assumed here that the circulating cooling water has a chloride ion content of 120 ppm. The temperature of the cooling water corresponding to different materials is not affected by corrosion. The 304 stainless steel is 25, 316 stainless steel is 62. It seems that the outlet temperature of 316 stainless steel for circulating water is generally 40 to 42 degrees Celsius is feasible.
As a material choice for the plate heat exchanger plate, if the cooling medium is ignored, only the chloride ion and temperature combination of the cooling water need to be considered. It is assumed here that the circulating cooling water has a chloride ion content of 120 ppm. The temperature of the cooling water corresponding to different materials is not affected by corrosion. The 304 stainless steel is 25, 316 stainless steel is 62. It seems that the outlet temperature of 316 stainless steel for circulating water is generally 40 to 42 degrees Celsius is feasible. -
However, it should be noted that in the heat transfer process of the plate heat exchange device, there is a film on each side of the metal plate, and there is a temperature difference between the heat transfer surface and the fluid itself, or the temperature of the metal wall of the heat exchange plate is higher than that of the plate heat exchanger. The temperature of the inner circulating cooling water, in other words, the temperature difference of the metal wall temperature of the heat exchange plate.If the temperature of the cold medium is higher, and the wall temperature of the plate of the heat exchanger is calculated to be higher than 62, the 316L stainless steel is not optional. At this time, it is necessary to consider stainless steel S31254 (or 254SMO) which is resistant to chloride ion corrosion.
-
If the circulating cooling water has a higher chloride ion content or a higher temperature, when the S31254 (or 254SMO) is still not enough, the plate material of the plate heat exchanger should be titanium.
-
Of course, with the improvement of materials technology, more of the conditions that are cooled are corrosive media, such as HCL, various acids and alkalis. This type of chemical equipment requires a large amount of special metals that are resistant to corrosion. The main metal materials commonly used in HY-industry plate heat exchangers are as follows:
-
Titanium plate and titanium-palladium alloy; super austenitic special stainless steel: 254SMO (UNS S31254), 904L (UNS N08904), AL6XN (UNS N08367); nickel-based alloy steel: Hastelloy C-276 (UNS N10276), C- 2000, D-205; low carbon pure nickel 201; Monel 400; austenitic stainless steel 304, 304L, 316, 316L, 317L, 310S, 321 and the like.Incidentally, the inner wall temperature of the plate heat exchanger is determined, preferably based on the temperature of the medium to be cooled and the temperature of the circulating cooling water. If the calculation is difficult, the design temperature of the equipment may be taken. Theoretical data is for reference, and more is actually the actual needs of the site. If there are conditions, you can do some hanging experiments for the actual working conditions, and meet the on-site service with the most economical materials. HY-industry can provide technical services if required.
3. The following are 13 kinds of commonly used plate heat exchangers and heat exchanger alloy materials:
-
254 SMO super stainless steel: excellent low chloride low grade stainless steel with improved Mo content to 6% and improved type 316, with excellent chloride pitting corrosion resistance And crevice corrosion performance, suitable for seawater, brine, inorganic acid and other media.
-
904L Super Stainless Steel: This is a cost-effective austenitic stainless steel that combines price and corrosion resistance. It has good corrosion resistance and is especially suitable for general acids and halides such as sulfuric acid and phosphoric acid (including Cl- and F-). ). Due to the high content of Cr, Ni and Mo, it has good resistance to stress corrosion, pitting and crevice corrosion. For some low-concentration sulfuric acid environments, some conditions can be used instead of 254SMO.
-
 Nickel 201: This is a pure nickel plate containing more than 99% nickel. It is mainly used in high concentration (50%~70%), high temperature (up to boiling point) caustic solution (NaOH, KOH, etc.). However, it should be noted that it is sensitive to crevice corrosion caused by chlorides such as brackish seawater.
Nickel 201: This is a pure nickel plate containing more than 99% nickel. It is mainly used in high concentration (50%~70%), high temperature (up to boiling point) caustic solution (NaOH, KOH, etc.). However, it should be noted that it is sensitive to crevice corrosion caused by chlorides such as brackish seawater. -
Hastelloy C-276: Has good corrosion resistance: it is hardly affected by chloride ions or chlorides; it has excellent corrosion resistance to various concentrations of sulfuric acid, and is one of the few materials that can be used for hot concentrated sulfuric acid; For organic acids (such as formic acid, acetic acid), high temperature HF acid and a certain concentration of hydrochloric acid (<40%), phosphoric acid (≤ 50%); chloride, fluoride and organic solvents (such as methanol, ethanol).
-
Monel 400: Ni (about 70%) – Cu (about 30%) nickel-based alloy. The corrosion resistance is good in a medium having a concentration of 80% or less, a temperature of not more than 50 to 100, a non-aerated sulfuric acid, a concentration of 50% or less, a temperature of 100 or less, and a medium such as acetic acid or caustic. It is especially suitable for acidic chloride solutions and brackish water and brine under certain working conditions; it has good high temperature resistance. However, it does not apply to concentrated sulfuric acid, hydrochloric acid and nitric acid, and is sensitive to the erosion of mercury (sometimes present as an impurity).
-
 Incoloy 825: Suitable for various concentrations of sulfuric acid at low temperatures; in a concentration of 50% to 70% of caustic (such as NaOH) solution, has good corrosion resistance, no stress corrosion cracking. However, it is sensitive to crevice corrosion caused by chloride. In addition, the stamping performance is not very good, so it is not a commonly used material for the sheet.
Incoloy 825: Suitable for various concentrations of sulfuric acid at low temperatures; in a concentration of 50% to 70% of caustic (such as NaOH) solution, has good corrosion resistance, no stress corrosion cracking. However, it is sensitive to crevice corrosion caused by chloride. In addition, the stamping performance is not very good, so it is not a commonly used material for the sheet. -
654 SMO Advanced Stainless Steel: This is an ultra-low carbon high grade stainless steel with Cr, Ni, Mo and N contents higher than 254 SMO. It has high MO and N content and is better than chloride 254. It can be used for 254 SMO. Cold sea water. PRE is 64. Performance is comparable to nickel based alloys.
-
Hastelloy C-2000: HASTELLOY C-2000’s most comprehensive corrosion-resistant alloy with excellent resistance to uniform corrosion in both oxidizing and reducing environments. It is also more resistant to localized corrosion and profitable corrosion cracking than the C-276 alloy.
-
Alloy 59: In the standard corrosion test of most oxidizing environments, Alloy59 nickel-based alloys have higher performance than other Ni-Cr-Mo stainless steels. Alloy59 nickel-based alloys are more than three times less corrosive in some reducing environments (such as boiling 10% sulfuric acid) than conventional Ni-Cr-Mo alloys, and are also suitable for use in the chemical process industry for reducing environments. Alloy59 nickel-based alloy has excellent corrosion resistance in hydrochloric acid.
-
Inconel 686 alloy: Similar to the corrosion resistance and application fields of the above 59, C2000 alloy, Inconel 686 alloy is the top commercial and patented product of nickel-based corrosion resistance of SMC in the United States.
-
Hastelloy BC-1: HASTELLOY HYBRID-BC1 (UNS N10362) alloy is a patented product of Hastelloys, USA. Compared with Hastelloy C series alloys, BC-1 has higher resistance to hydrochloric acid and sulfuric acid corrosion and is extremely resistant. Pitting corrosion and crevice corrosion. Hastelloy BC-1 alloy is resistant to reductive acid corrosion in an oxidizing environment up to 427 ° C (800 ° F).
-
Titanium, GR.1: Unalloyed titanium, light weight, density 4.51, can naturally form a passivation protective film (Ti2O3), and if it is destroyed, it has “self-healing”, so the corrosion resistance is better than stainless steel. A typical material suitable for chlorine-containing media (Cl-concentration >200mg/L, temperature ≤130). In seawater and other chloride (such as CaCl2) solutions of no more than 120, it is virtually uncorroded. It is generally used for seawater below 135 and brine (NaCl) at various concentrations below 165. Titanium has good corrosion resistance in organic acids (such as concentrated nitric acid, concentrated carbonic acid, etc.) and dilute lye below the boiling point. Titanium has poor corrosion resistance in H2SO4, HCl, HF, and aqua regia. In some concentrated chloride solutions (such as pH >7, chloride concentration >200mg/L) at high temperatures (above 120), crevice corrosion or stress corrosion may also occur. At this time, a titanium-palladium alloy should be used.
-
Titanium-Palladium Alloy, GR.11: This is a non-alloyed titanium with added palladium (0.12% to 0.25%), thus significantly improving the corrosion resistance of titanium in an acid medium. For example, it has good corrosion resistance to nitric acid having a concentration of 70%, hydrochloric acid containing oxidizing ions (such as Fe+, Cu+), and plating solution. In addition, it can be used for dilute sulfuric acid with a concentration of ≤10% and a temperature of ≤70.