Material selection in high hydrogen sulfide environment.(HY-industry technical centre)
titanium alloys
Inconel 625
Incoloy 825
HASTELLOY C-276
Ti 6Al-2Sn-4Zr-2Mo
1 Introduction
 With global energy demand growing and oil resources depleted, oil reserves are deeper and more difficult to mine. With existing technology, the well can be mined to more than 35,000 feet. To this end, the oil and gas industry has developed a large number of proprietary technologies over the past few decades. The materials required for extreme conditions have always restricted the exploitation of deep well fields, especially acidified oil fields containing hydrogen sulfide (acid gas).
With global energy demand growing and oil resources depleted, oil reserves are deeper and more difficult to mine. With existing technology, the well can be mined to more than 35,000 feet. To this end, the oil and gas industry has developed a large number of proprietary technologies over the past few decades. The materials required for extreme conditions have always restricted the exploitation of deep well fields, especially acidified oil fields containing hydrogen sulfide (acid gas).
Simulating acidified oil well environments is a common practice in the oil and gas industry. This technique pumps a strong acid through the pipeline into the reservoir. Commonly used acids are hydrochloric acid, hydrofluoric acid, acetic acid and formic acid. These acids are highly corrosive to every alloy used in production, and the acid rate increases sharply in high temperature and high pressure environments.
Acidification is a common practice in oil production by pumping strong stimulating acids into the reservoir through pipes, avoiding damage to the formation, increasing the porosity of the formation, and cleaning up sediments in the pipeline. Concentrated inorganic and organic acids containing preservatives are used for acidification. Commonly used solutions include:
-
10% hydrochloric acid
-
15% hydrochloric acid
-
28% hydrochloric acid
-
12% hydrochloric acid / 3% hydrofluoric acid
-
15% hydrochloric acid / 10% acetic acid
-
10% acetic acid
-
10% formic acid
In high temperature acidification solutions, corrosion damage can occur even with the addition of large doses of suitable corrosion inhibitors. The basic rule for high temperature acidification solutions is that corrosion rates below 2000 mpy (50.8 mm/year) are considered acceptable depending on the period of use of the pipe in the acid solution.
In order to prevent excessive damage or corrosion of the ground formation, organic acid solutions are sometimes used. 10% acetic acid or 10% formic acid solution is the most commonly used organic acidification solution. The results show that these organic acid solutions are much less corrosive than inorganic acids, especially for corrosion resistant alloys. For example, Ti Beta-C has a zero corrosion rate of 10% acetic acid or 10% formic acid at 232 °C.
In a certain temperature range, corrosion inhibitors can provide sufficient corrosion protection for different grades of alloy pipes. Studies on inorganic acids with corrosion inhibitors at elevated temperatures included evaluation of the following alloys: N80 carbon steel, 13 chrome martensitic stainless steel, duplex stainless steel, nickel based alloys and titanium alloys.
 Laboratory and field experiments have found that corrosion inhibitors have limited effects. The on-site use of 25Cr duplex stainless steel pipe shows that the corrosion rate is about an order of magnitude higher than the corresponding laboratory data at temperatures around 130 °C; this finding means that the corrosion inhibitor performance at the site is reduced because the flow rate is considered. Or the consumption of corrosion inhibitors. A number of studies have concluded that corrosion inhibitors have different anti-corrosion effects between different types of alloys, such as carbon steel and duplex stainless steel, and it is difficult to alleviate corrosion of petroleum pipelines composed of different alloys. The presence of hydrogen sulphide in many wells also affects the preservative effect, and there may be a “competition” between the sulphide and preservatives on the alloy surface, which increases the corrosion rate by an order of magnitude.
Laboratory and field experiments have found that corrosion inhibitors have limited effects. The on-site use of 25Cr duplex stainless steel pipe shows that the corrosion rate is about an order of magnitude higher than the corresponding laboratory data at temperatures around 130 °C; this finding means that the corrosion inhibitor performance at the site is reduced because the flow rate is considered. Or the consumption of corrosion inhibitors. A number of studies have concluded that corrosion inhibitors have different anti-corrosion effects between different types of alloys, such as carbon steel and duplex stainless steel, and it is difficult to alleviate corrosion of petroleum pipelines composed of different alloys. The presence of hydrogen sulphide in many wells also affects the preservative effect, and there may be a “competition” between the sulphide and preservatives on the alloy surface, which increases the corrosion rate by an order of magnitude.
Uniform corrosion is the most common form of acidified solution erosion, as well as localized and stress corrosion cracking. Although most laboratory acidification experiments were carried out without hydrogen sulfide, tests to evaluate the effects of hydrogen sulfide have shown that hydrogen sulfide has a strong influence on uniform corrosion, local corrosion and stress corrosion cracking. Figure 1 is a set of data from Kane and Wilhelm. It can be seen that the uniform corrosion rate of various materials after adding hydrogen sulfide to 15% hydrochloric acid containing corrosion inhibitors is increasing. Kolts and Corey’s research found that a variety of nickel-based alloys exhibit stress corrosion cracking under acidification conditions with added corrosion inhibitors and hydrogen sulfide, and the same alloys do not appear under the same conditions without hydrogen sulfide. fracture.
In the acidified solution containing no corrosion inhibitor, the corrosion is the most serious. This environment can more accurately reflect the acidification operation in the flow state or the corrosion inhibitor is consumed in the shallow layer of the well. As mentioned earlier, the corrosion rate in the acidified solution is high even at moderate temperatures. Garber and Kantou found that the corrosion rate of alloy Inconel 625 in 10% hydrochloric acid solution without corrosion inhibitor was from 15 mpy (0.4 mm/y) at 40 °C to 300 mpy (7.6 mm/y) at 82 °C. Corrosion resistant titanium alloys, such as Ti Beta-C, have a corrosion rate of 584 mpy (14.8 mm/y) in a 10% hydrochloric acid solution at 100 °C. Regardless of whether or not a corrosion inhibitor is added to the acidifying solution, the corrosion rate increases remarkably as the temperature rises. It was found by experiments with 15% hydrochloric acid at a high temperature of 177 ° C and no corrosion inhibitor that nickel-based alloy 535, Incoloy 825 and HASTELLOY G3 were completely dissolved.
This paper focuses on the results of laboratory studies to evaluate the corrosion resistance of various alloys in the acidification environment of oil and gas exploration in deep wells, and to help users in the oil and gas industry to further understand the materials applicable to these extreme conditions. There are several alloys used in the experiment, including stainless steel (316L), nickel-based alloy (HASTELLOY C-276), titanium alloy (Ti6 and Ti 6Al-2Sn-4Zr-2Mo), and bismuth composite (316L+ bismuth alloy surface). ). The test was carried out in two groups. The acidification environment was divided into weak (10% acetic acid) and strong (10% hydrochloric acid + 10% acetic acid + 15 psia hydrogen sulfide), all of which contained no corrosion inhibitor. The test temperature was 232 ° C, which was equivalent to the deep well. Bottom temperature.
2, the problem
In order to carry out the test under the specified environmental conditions, the 0.5L autoclave made of HASTELLOY C-276 alloy was initially selected and equipped with normal fittings such as thermocouples, valves and fasteners. Due to the corrosive nature of the test environment, the integrity of the HASTELLOY C-276 reactor exposed to multiple corrosive environments has been problematic. The wall thickness of the autoclave was greatly reduced, which eventually led to a catastrophic accident in the pressure vessel. In addition, the dissolution of the test equipment caused pollution to the test environment, causing doubts about the validity of the test results.
2.1 test
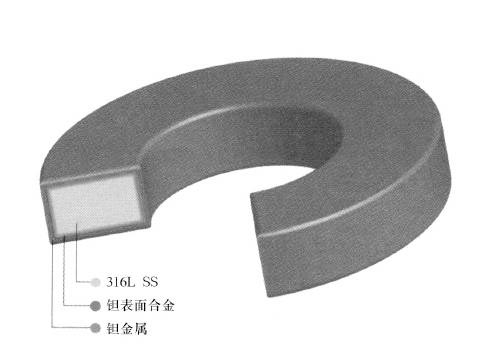
The acidified exposure test was carried out in a static degassing environment. Initially, the 0.5L autoclave was made of HASTELLOY C-276 alloy and equipped with normal fittings such as thermocouples, valves and fasteners. Due to the corrosive nature of the test environment, problems with the integrity of the HASTELLOY C-276 alloy reactor end up. The wall thickness of the autoclave was greatly reduced, which eventually led to a catastrophic accident in the pressure vessel. In addition, the dissolution of the test equipment caused pollution to the test environment, causing doubts about the validity of the test results. Due to the strong corrosive nature of the environment, it is necessary to prevent excessive corrosion and possible pressure vessel failure. The material of the autoclave is changed to a 316L composite plate whose surface is niobium alloy. The components in the autoclave were made of niobium alloy + 316L composite or alumina ceramic. The results show that the niobium surface alloy plays a key role in the integrity of the equipment in the harsh acidification test.
2.2 Materials and test conditions
Five materials were evaluated in this study. They were 316L, HASTELLOY C-276, Ti 6-4, Ti 6Al-2Sn-4Zr-2Mo, and tantalum surface 316L. Two samples were tested for each alloy. Both test conditions were 232 ° C without corrosion inhibitor acidification solution. Acidification fluids with corrosion inhibitors were used in both laboratory and field tests. These corrosion inhibitor formulations are for specific alloy types (eg carbon steel, nickel alloys, etc.) as described in the introduction. The purpose of this study was to evaluate the most demanding environments (no corrosion inhibitors) and the extent to which various alloys were corroded. No corrosion inhibitors were used in each test.
3, the results
The corrosion rates measured from the two sets of experiments are quite different. The corrosion rate of 10% acetic acid at 232 °C is mild, and the corrosion rate of 316L is 242 mpy (6.1 mm/y). In the environment of 10% hydrochloric acid/10% acetic acid and containing hydrogen sulfide, except for 316L on the surface of the crucible, the corrosion rate of all other alloys is very high. After 8 hours of exposure, 316L, Ti 6-4, Ti 6Al-2Sn- The 4Zr-2Mo sample was completely dissolved, and the HASTELLOY C-276 sample was severely corroded.
3.1 test results: 10% acetic acid, 232 ° C
The corrosion rate of the test alloy in 232 ° C 10% acetic acid was from zero to light as expected in the previous test in a high temperature organic acid acidification solution. The corrosion rate of the niobium surface alloy and Ti 6-2-4-6 was zero. The corrosion rate of 316L was 242 mpy (6.1 mm/y), and the corrosion of HASTELLOY C-276 and Ti6-4 was 11.9 mpy (0.3 mm/y) and 2.2 mpy (0.1 mm/y), respectively. According to the visual inspection, the surface of the niobium surface alloy device was not affected at all.
In the 232 ° C 10% hydrochloric acid / 10% acetic acid / 15ps hydrogen sulfide test, in addition to the surface alloy, the corrosion rate of other tested alloys is very high. The corrosion rate of the niobium surface alloy sample was zero, and only the blue film produced from the metal ion plating in the solution during the exposure period was observed. After 8 hours of exposure to the solution, the 316L, Ti 6-4, Ti 6-2-4-6 samples were completely dissolved (see photo of the test frame after exposure in Figure 5), indicating a corrosion rate of at least 16 inches/year. (406mm / y) ~ 41 inches / year (1049mm / y). The HASTELLOY C-276 specimen was severely corroded with a corrosion rate of 21 inches/year (531 mm/y). According to the naked eye inspection, there is no trace of corrosion on the surface alloy equipment and the configuration parts.
The usability of niobium surface alloy samples, autoclaves and fittings proves that in the strong erosion environment test, the anti-corrosion effect of the treated surface is very good. After inspection of the sample, the kettle and the fittings, no localized erosion, air bubbles or other damage was observed in the surface alloy or the underlying base metal.
4, summary
In high temperature hydrogen sulfide containing environments, conventional high performance corrosion resistant alloys are severely corroded. This means that in special applications where other materials do not meet the requirements, niobium surface alloys exhibit extraordinary corrosion resistance.
Due to their unmatched corrosion resistance, availability and economy, niobium surface alloys are expected to replace high-performance corrosion-resistant alloys such as nickel, titanium, zirconium and, of course, niobium alloys.